- News
- lifestyle
- health-fitness
- health-news
- Medical Breakthrough: 1st lung cancer vaccine BNT116 clinical trials launched, vaccine based on Covid mRNA
Trending
Medical Breakthrough: 1st lung cancer vaccine BNT116 clinical trials launched, vaccine based on Covid mRNA
BioNTech has launched global clinical trials for BNT116, an mRNA vaccine designed to treat non-small cell lung cancer. Utilizing the same mRNA technology as Covid-19 vaccines, BNT116 aims to prime the immune system to identify and attack cancer cells. The trial will include about 130 patients across seven countries.
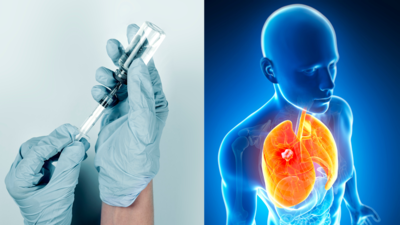
Unlike traditional viral vaccines, BNT116 aims to activate the immune system to recognize and attack lung cancer cells. This is achieved by exposing the immune system to tumor markers specific to NSCLC.

The trial will take place in seven countries with around 130 participants who have different stages of lung cancer, ranging from early to advanced or recurrent forms. Participants will receive BNT116 in combination with immunotherapy, which may enhance the treatment's effectiveness.
A significant advantage of BNT116 is its potential to reduce lung cancer recurrence, thereby possibly improving long-term survival rates. Researchers will assess the vaccine’s effectiveness in preventing lung cancer relapse throughout the trial. Positive outcomes could establish mRNA cancer vaccines as a standard treatment option globally.
In June, the US Food and Drug Administration placed a partial clinical hold on BioNTech’s Phase I trial of BNT326/YL202, an early-stage antibody-drug conjugate, after deaths were reported.
This development in mRNA vaccine technology highlights its potential beyond viral infections, aiming for groundbreaking cancer treatment advancements.
5 Exercises for quick weight loss

About the Author
TOI Lifestyle DeskEnd of Article
FOLLOW US ON SOCIAL MEDIA








